
 |
| | |||||
| Part 1 | Relaxation | Theory (III) | |||
| Part 2 | Pulse sequences | ||||
| Part 3 | Spin echoes | ||||
| Imaging parameters | |||||
| Special applications | |||||
| References | |||||
|
Relaxation |
||
|
Relaxation is unavoidable if the system under investigation is perturbed by a radio frequency pulse and therefore moved away from equilibrium. We have to consider two different processes: T1 and T2 relaxation. The former is also called "spin-lattice relaxation", since there is some interaction with the environment which enables energy loss of the spins by transition from the excited to the ground state. The released energy can be absorbed by surrounding molecules in terms of kinetic energy. The resulting increase in ground state occupation leads to a rebuilding of the magnetization component along B0 (the so-called longitudinal magnetization) thereby approaching M0 (see Fig. 3).
On the other hand there is no change in energy during T2 relaxation (spin-spin relaxation) but rather in the phases of individual spins. This is due to fluctuations of the local magnetic field caused by the surrounding molecules. The resulting spin dephasing leads to a decrease of transverse magnetization Mxy (magnetization component in the xy-plane) and therefore to a signal decay according to Fig. 7. The described mechanisms are displayed in Fig. 14. |
||
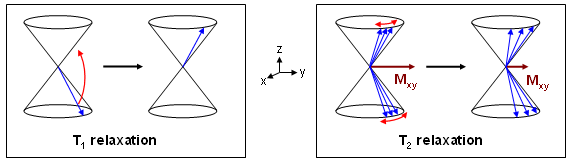 Figure 14 |
From this figure it is clear that the energy change during T1 relaxation occurs without phase alterations (component in xy-direction remains constant), whereas T2 relaxation results in a phase change only. Therefore, both processes are independent of each other and their time responses can be described separately, even though both mechanisms proceed simultaneously. Thereby, they cause a complex trajectory of the total magnetization vector during relaxation (alterations in direction and magnitude). |
|
Time courses of the magnetization for T1 and T2 relaxation are shown in Fig. 15 for fat, blood and water. t=0 is the time point immediately after a 90° excitation pulse with the curve starting at a relative intensity of 1. As can be seen from the equations the relaxation behaviour can be described by exponential curves that can considerably vary for different matters, depending on the size of the time constants T1 and T2.
For example for water, both T1 and T2 are quite high, so that rebuilding of the longitudinal as well as dephasing of the transverse magnetization are slow. For fat, however, both relaxation processes are considerably faster. |
 Figure 15 |
| All relaxation mechanisms mentioned so far are heavily influenced by temperature and molecular environment. By contrast, the so-called T2* relaxation (as a variant of T2) is a result of dephasing processes due to an inhomogeneous magnet field which can be minimized by manual justification ("shimming"). Since T2* is usually much smaller than T2, the signal decay of an FID (see Fig. 7) is almost completely caused by T2* effects. In general, T1>T2>T2*. |
| Top |
Pulse sequences |
|
A pulse sequence is the implementation of the hardware components necessary for excitation, spatial encoding, and data acquisition in MR imaging. It is described by a scheme in which all RF pulses and gradient amplitudes as well as the acquisition window are displayed as functions of time for the smallest repetition interval. This will be explained in detail for the pulse sequence of a simple 2D gradient echo method (Fig. 16). The upper trace (RF) shows the radiofrequency excitation pulse with a variable flip angle α which is usually considerably smaller than the 90° shown in Fig. 4 (see below). The three traces indicated by SS, PE, and RO represent the individual gradient amplitudes, ACQ shows the acquisition window.
|
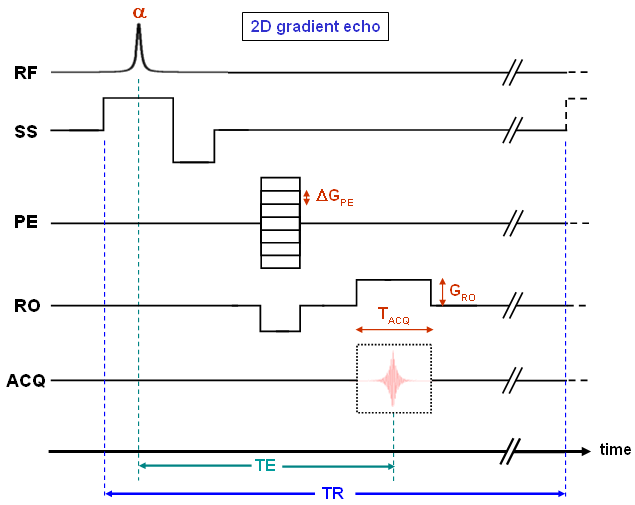 Figure 16 |
Slice selection (SS) is realized by switching on the appropriate gradient at the same time as the RF pulse. This pulse only excites spins within a defined interval of the total magnetic field (B0+zSS·GSS). Since different phases are generated within the excited slice by the SS gradient, a rephasing pulse with negative amplitude is necessary to compensate for this effect. Subsequently, phase encoding (PE) and readout dephasing gradient (RO) are switched on and off before GRO is applied simultaneously with data acquisition. It must be noted that due to the orthogonality of the three directions, all phase manipulations can be applied independently. Hence, GPE and the negative readout gradient can also be switched at different times. Alternatively, both gradients can be applied at the time of slice selection rephasing. These details depend on the implementation of the pulse sequence or on the timing parameters selected by the user. By contrast, differences specific to the individual sequences are mentioned explicitly. |
| The timing diagram shown in Fig. 16 covers the acquisition of one k-space line which is repeated (almost identically) until the end of the measurement. Thereby, the RF pulse and the gradients in slice selection and readout directions remain unchanged, whereas the phase encoding gradient is incremented by a constant amount ΔGPE between the cycles. This leads to a depiction of GPE according to Fig. 11 (left) within the pulse sequences. |
|
In figure 16 we see two very important timing parameters: the echo time (TE) and the repetition time (TR). TE is defined as the time between excitation and maximum amplitude of the echo, TR is the duration of a phase encoding cycle. Both quantities are imaging parameters defined by the user (see below), and they can be used e.g. to produce contrast between different tissues due to their individual relaxation properties. As indicated by the axis breaks, the length of TR can be raised to any value by increasing the time interval between data acquisition and the next excitation pulse.
Most pulse sequences are variants of the gradient echo or the spin echo method. The (2D) sequence of the latter technique is displayed in Fig. 17 and its principles are described in the next section. |
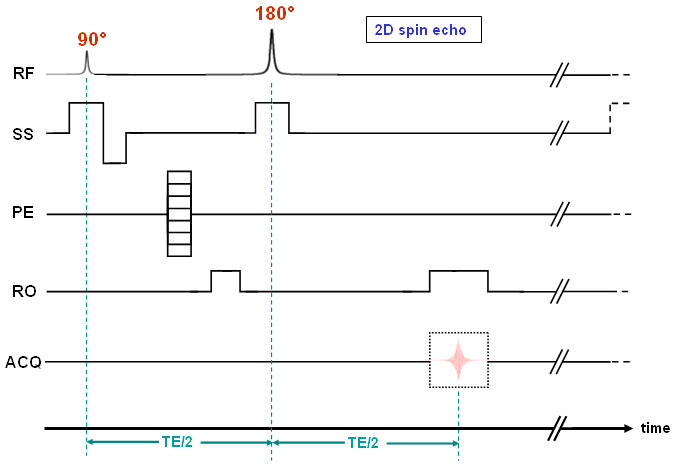 Figure 17 |
| Top |
Spin echoes |
| As illustrated in Fig. 17 we need two RF pulses within a spin echo cycle, the first one for excitation and the second one for the so-called refocussing. While the flip angle (α) is usually lower than 90° in gradient echo sequences, we use a 90° pulse for excitation and an exact 180° pulse for refocussing for the spin echo method. |
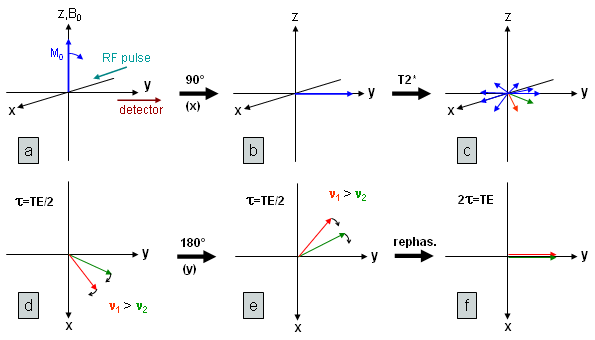 Figure 18 |
Fig. 18 will help us to understand this: Here, we see the excitation (a,b) and the subsequent dephasing of the spins (c) destroying the transversal magnetization. This is mainly due to magnetic field inhomogeneity (T2* relaxation) which is responsible for the fast decay of the FID. In (d) two vectors are extracted representing spin ensembles with two different frequencies at the time τ after excitation (shown is the xy plane only). The refocussing pulse (at time τ=TE/2) in the y-direction leads to a 180° rotation about this axis (e). Thereby the phase is inverted, but the angular velocity (frequency) that is determined by the local magnetic field does not change (the direction of rotation is also not altered because it is defined by the difference between actual and reference frequency). Therefore, the vectors are now moving towards the y-axis with the faster "lying behind" the slower one. TE/2 after the refocussing pulse both vectors will coincide and are so refocussed on the y-axis (f). |
|
Of course, this principle can be applied to an arbitrary number of vectors, so that all dephasing partial magnetizations can be refocussed at one time thereby leading to a so-called spin echo.
In general, gradient echos are produced rapidly after excitation, that is de- and rephasing by application of the respective gradients take place during the free induction decay. By contrast, refocussing can be applied some time after the FID using spin echo sequences. As long as the local magnetic field of the spins does not change, the procedure can be repeated until the signal has completely decayed due to T2 relaxation. In Fig. 19 two echoes are shown with their maxima exponentially decreasing according to spin-spin relaxation law. T2 relaxation is very fast, i.e. the molecular environment of the spins is changing very rapidly. As a consequence, T2 effects are not affected by the spin echo procedure and can therefore not be compensated by a refocussing pulse. |
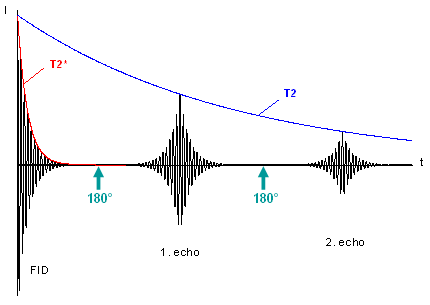 Figure 19 |
| Finally, here are some comments concerning Fig. 17: Since the 180° pulse must be applied to the same slice as the excitation, the slice selection gradient must be switched on at the same time as the refocussing pulse. A subsequent rephasing is not necessary, because GSS is placed symmetrically around the 180° pulse and therefore, phase shifts produced before and after the pulse should completely cancel out (in analogy to the situation shown in Fig. 18d-f). This also explains the positive dephasing gradient in the readout direction. |
| Top |
Imaging parameters |
| Since there is a multitude of parameters in MR imaging some of which are specific to the pulse sequence applied, we can only choose some of them for the following discussion. An important parameter that has been mentioned several times in the previous sections is the flip angle (α). This is used to manipulate intensity and contrast in the final image. |
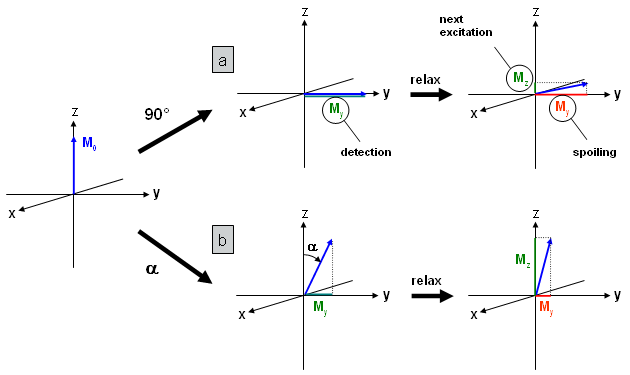 Figure 20 |
In Fig. 20a a 90° pulse and subsequent relaxation is shown for a gradient echo sequence. As already mentioned this sequence can be applied with very short repetition times, so that the time between data acquisition and excitation pulse of the next phase encoding cycle is also very short. Hence, only a small fraction of the longitudinal magnetization is restored by T1 relaxation (Mz, green).
Residual transversal magnetization (My, red) is usually destroyed by so-called spoiler gradients and therefore not available for subsequent excitations. Hence, there is only a small amount of magnetization that can be used in the following phase encoding cycles leading to weak intensities in the MR image. |
|
In Fig. 20b the situation is shown for a flip angle smaller than 90°. After the first excitation pulse the signal at the detector (My=sin(α)·M0, green) is smaller than in the 90° case. However, after relaxation, the longitudinal component Mz which is used for subsequent phase encoding cycles is considerably larger. Therefore, using a flip angle smaller than 90° (obtainable by a reduced pulse duration or intensity) leads to higher signal intensity. For each material (tissue) and repetition time the flip angle can be optimized to provide maximum intensity (→ Ernst angle). The method described is usually called FLASH (Fast Low Angle SHot) or GEFI (Gradient Echo Fast Imaging) sequence. |
|
Sometimes we are interested in a maximum contrast between two adjacent matters rather than in maximum intensity. This can be produced by taking advantage of different relaxation properties. For example, a short repetition time usually leads to a T1-weighted image, since longitudinal relaxation after excitation can have different courses depending on the respective T1 constants.
In the top of Fig. 21 we see the T1 relaxation curves for blood and fat which display the relative longitudinal magnetization (i.e. related to M0) after a 90° excitation. Additionally, the black difference curve indicates that the maximum intensity difference can be found at a relatively short TR of 300 ms. For t=T1 (dotted line) the transversal magnetization has increased to the value 1-1/e (63 %). To (almost) completely exclude T1 weighting, at least TR=5·T1 should be used (i.e. about 4 seconds for blood). If, at the same time, short echo times are used in order to minimize dephasing effects, we obtain proton density- (PD-) weighted images which simply means that high signal is equivalent to a high number of protons. By contrast, by using long echo times we can enhance contrast due to different T2 relaxation properties of the matters involved. In the bottom half of Fig. 21 T2 curves for blood and fat are displayed as well as the difference plot showing a maximum at 125 ms which is a quite long echo time. For t=T2 the signals have dropped to a fraction of 1/e (37 %). It should be mentioned that T2-weighted images are inaccessible by the FLASH sequence, since a fast signal decay is induced by T2* relaxation. Hence, refocussing spin echo methods are needed to realize long echo times. |
 Figure 21 |
 Figure 22 |
TR-TE combinations are summarized in the table of Fig. 22 which also shows some brain images impressively demonstrating the effects of relaxation weighting on the contrast. A specific weighting for the combination short TR/long TE cannot be defined. This parameter combination is in any case impractical due to a very low signal-to-noise ratio (SNR).
The three parameters discussed so far are summarized in Tab. 1 together with several other important variables. The field of view (FOV) defines the image size in two (for 2D scans) or three (for 3D scans) dimensions and is either given as the product of all lengths or as one defined length indicated by a subscript (as listed in Tab. 1 for readout and phase encoding directions). In the 3D case the FOV of the third dimension corresponds to the slice thickness in a tomographic image. The spatial resolution along this direction is obtained by additional phase encoding (called partition encoding for the third dimension) steps. We can determine the pixel (2D) or voxel length (3D), respectively, in each direction by the ratio FOV/number of points. These lengths define the spatial resolution of the image. The number of points of the raw data matrix does not necessarily match the number of pixels in the final image. In fact, the size of k-space can be easily increased by filling it with zeros (therefore this procedure is called zero filling) also leading to an increased image size after Fourier transformation. Hence, this process apparently increases the image resolution by means of a complex averaging operation. |
|
|
The number of scans (NS) can be increased in order to improve the signal-to-noise ration. Unfortunately, the total acquisition time is proportional to the number of scans, whereas SNR is improved only by the square root of NS.
The band width corresponds to the sampling rate during data acquisition. Hence, its reciprocal value is the time Δt between the registration of two data points. Multiplication of Δt with NRO provides the length of the acquisition window (TACQ). The total acquisition time required for a 2D image can be calculated from Ttot = NS·NPE·TR. For 3D scans we additionally have to multiply this value with the number of partition encoding steps. |
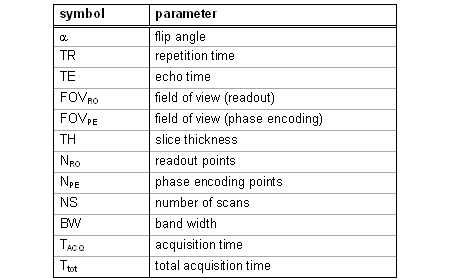 Table 1 |
| Top |
Special applications |
| All MR imaging techniques can be assigned to either gradient echo or spin echo methods (see Fig. 16 and 17). However, there are countless variants usually specialized for the measurement of certain chemical or physical properties. Methods like chemical shift or diffusion-weighted imaging which are applied in our laboratory are discussed in detail in this section. |
| CSI (Chemical Shift Imaging) |
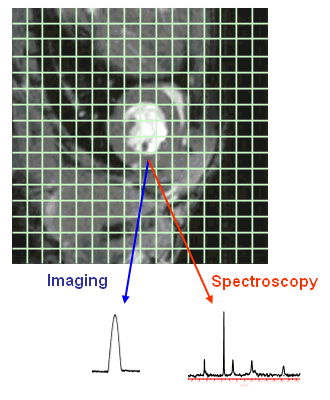 Figure 23 |
CSI is a combination of MR imaging and NMR spectroscopy and is therefore also called "spectroscopic imaging" or "localized spectroscopy".
In tomographic images a signal can be assigned to each pixel after two-dimensional Fourier transform of the raw data. The integral of the signal is proportional to the intensity (grey scale value) in the image. In CSI a complete NMR spectrum is assigned to each pixel instead of a single value, but the grid is usually much coarser, so that the number of pixels reduces significantly. This is illustrated in Fig. 23 which shows the tomographic 1H-MR image of a mouse heart with a superimposed 16×16 grid. The cardiac image consists of 256×256=65536 pixels, so that in each of the green squares there are 256 pixels with individual grey scale values. For each of these squares the CSI method provides a spectrum (in the figure a 31P spectrum is shown with signals for inorganic and high-energy phosphates, such as adenosine triphosphate and phosphocreatine) and hence, we obtain a data set consisting of 256 spectra. Such a set of spectra can be used for e.g. the characterization of the energy state of a mouse heart in vivo. By using this method signals that stem from outside the heart can be eliminated and regional differences within the heart may be detected. |
|
To obtain the spectroscopic information within the FID or echo, we cannot use frequency encoding to encode spatial information (as in standard MR imaging methods) and therefore, no readout gradient is used (see Fig. 24). As in NMR spectroscopy an FID is acquired with frequency components stemming from nuclei with different environments.
Using no readout gradient means to spatially encode with phase encoding steps only, so that for an N×N matrix N2 measurement cycles are required. This is one of the reasons for the considerably lower matrix size in CSI applications compared to standard imaging data sets. In summary, a (2D) CSI sequence provides three-dimensional data sets with two dimensions for phase encoding of the spatial coordinates and one for the spectroscopic component. |
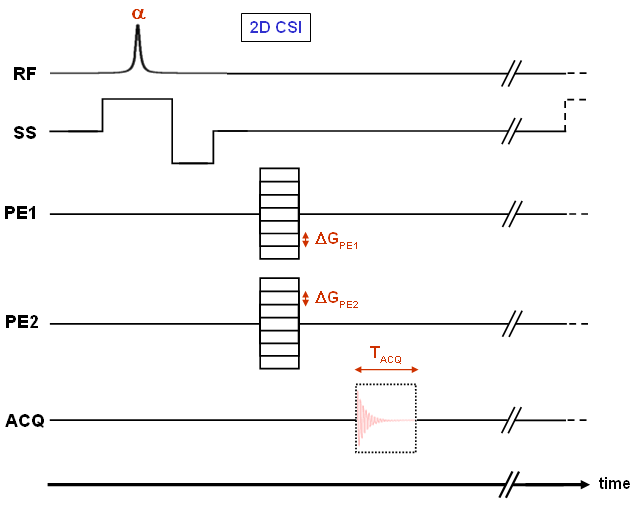 Figure 24 |
 Figure 25 |
| Using a double-tuned probe head, we can consecutively perform 1H and 31P measurements of the same slice without modification of the set-up. Hence, the anatomical reference for the 31P-spectra can be reliably obtained as shown in Fig. 25. In this screenshot of the in-house-developed program "CSI-Tool" we see the tomographic image of a midventricular short axis slice (pilot scan, left) as well as the spectra of the green square areas (CSI scan, right). By means of this software we can e.g. add and/or integrate the spectra assigned to different regions (Further information is available here). |
| Diffusion-weighted imaging |
| Diffusion-weighted (spin echo) sequences are widely-used in medical applications, since e.g., they can be used for very early detection of infarcted areas in the brain (stroke diagnostics). They are accompanied with a signal decrease at places of high diffusion and a relative increase in areas where motion is hindered. |
|
To explain the principle of diffusion weighting, we first look at the bipolar pulse in Fig. 26a which can be integrated into a gradient echo sequence. All spins are affected by the positive gradient in such a way that they obtain a phase which is proportional to the area of the gradient pulse (here G·δ) and which is specific for each position along the gradient axis. The effect of the first pulse is compensated (the magnetization is refocussed) by the subsequent negative pulse of same area, provided that the spins are still at the same place.
All spins that have moved in the meantime (along the gradient direction) are not refocussed and therefore lost for signal formation. The greater the degree of diffusion, the greater the amount of non-refocussed spins and subsequently the intensity decreases. Relative to the areas of strong diffusion, pixels with reduced motion appear to be bright, since there is less intensity decrease for these pixels (compared to the image without a bipolar pulse). The extent of signal decrease can be described by the exponential equation S=S0·exp(-b·D), where S0 is the signal with gradients off (for identical echo time!), D the diffusion constant, and b the so-called b-factor which depends on the experimental parameters. For the bipolar pulse in Fig. 26a, b can be calculated by b = ²/3·γ2·G2·δ3. Increasing G or δ are both accompanied by an enhanced intensity loss. |
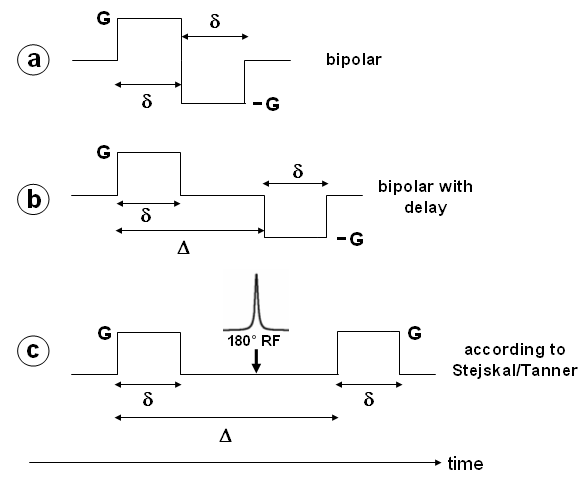 Figure 26 |
| The flexibility of the sequence can be increased by the introduction of an additional delay Δ (see Fig. 26b) that defines the time between the two gradient pulses. Incrementing this delay also leads to an intensity loss in the presence of diffusion, but is also accompanied by a relatively long echo time. In gradient echo sequences this leads to a strong signal decrease due to T2* relaxation and therefore to a small S0. Hence, it is advantageous to use spin echo sequences with the refocussing pulse now flanked by the diffusion sensitive gradients (Fig. 26c). Note that, due to the 180° pulse, the gradients are equal in sign. This sequence was initially published by Stejskal and Tanner (J Chem Phys 42, 288 (1965)), and the equation for b is, as for the bipolar pulse experiment in Fig. 26b: |
| b = γ2·G2·δ2(Δ–¹/3δ) |
| Using this pulse sequence, it is possible to either produce contrast between high and low diffusion areas (diffusion-weighted imaging) or determine a diffusion constant for each pixel and display a magnitude image of these constants. Since in vivo molecular diffusion is always restricted, they are called apparent diffusion constants (ADCs), and the resulting image is named ADC map. |
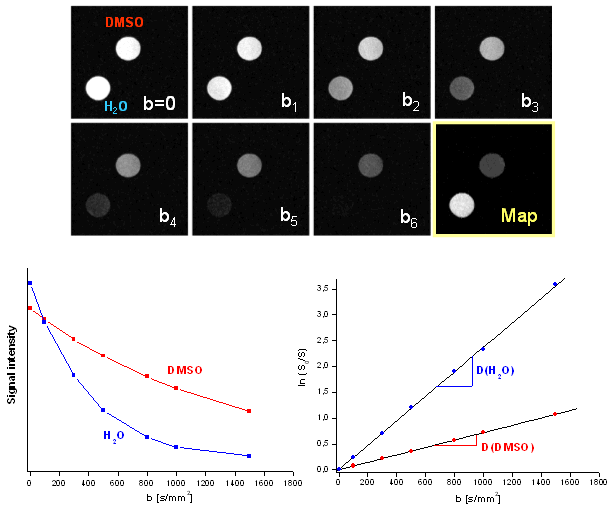 Figure 27 |
This is shown in Fig. 27 with a normal spin echo image (b=0) of two phantoms, dimethylsulfoxid (DMSO) and water, on the top left. The following images named b1 to b6 clearly show the effect of the diffusion gradients during successive increase of the gradient amplitude (G) and therefore of b (with constant delays δ and Δ). According to the exponential decays shown at the bottom left, the signal intensity of water shows a stronger decrease than DMSO with increasing b and therefore, the contrast between these two probes is continuously enhanced. From the exponential curves, one can easily deduce the ADCs of water and DMSO. In this case, they are identical to the diffusion constants (D) since the molecular motion is not restricted e.g. by cellular membranes. Displaying the logarithmic form ln(S0/S) as a function of b (shown on the bottom right of Fig. 27) is more convenient, since the slope of the regression line is identical to the diffusion coefficient. For this analysis we used an intensity averaged over the area of the respective phantom. The determination of D can also be performed on a pixel-to-pixel base. These diffusion constants can be displayed as grey scale values, and we obtain an ADC map like the yellow-framed image in the figure. Note that for such a diagram, several experiments (with different values for b) are necessary. |
| In the previous experiments, we did not define any direction of the diffusion gradients. This is not necessary if we assume a spherically symmetric diffusion behaviour. However, looking at a system with anisotropic diffusion, the gradient direction becomes very important. Therefore, scans with (at least 6) different gradient directions have to be performed to obtain adequate spatial information. From these experiments the so-called diffusion tensor can be determined which contains the diffusion constants for the individual directions. This method named Diffusion Tensor Imaging (DTI) provides an ADC matrix instead of a single constant. By diagonalization of the matrix we obtain the eigenvalues which are identical to the magnitudes along the principal axes of the diffusion ellipsoid. The directions of the principal axes are the eigenvectors of the tensor also obtained from the diagonalization procedure. |
|
This is shown in Fig. 28 which (at the top) displays the system of equations that is the tensor form of the one-dimensional approach S=S0·exp(-b·D). Since the problem is symmetric, both tensors, b and D, are symmetric as well and therefore, the (maximum) number of different tensor elements Dij is 6. These elements can be determined by 6 different experiments with linearly independent gradient directions, since this leads to 6 equations for the calculation of 6 unknowns. As above, an additional measurement with |b|=0 is necessary for the determination of S0.
Usually, the principle axes of diffusion that build up an ellipsoid are not parallel to the laboratory axes xyz, so that the non-diagonal elements of the diffusion tensor are non-zero. Diagonalization of the matrix is equivalent to a change into the principal axes (=eigenvectors) system and the diagonal elements are proportional to the magnitude of diffusion along the respective directions. In principle, you can determine such an ellipsoid for every pixel (analogous to the ADC map mentioned above), but this obviously leads to a confusing representation. Therefore, in the majority of cases diffusion images show either the magnitude or one component of diffusion. Another widely-used method called "fiber tracking" uses three-dimensional lines constructed from the directions of the highest eigenvalues of the individual pixels. |
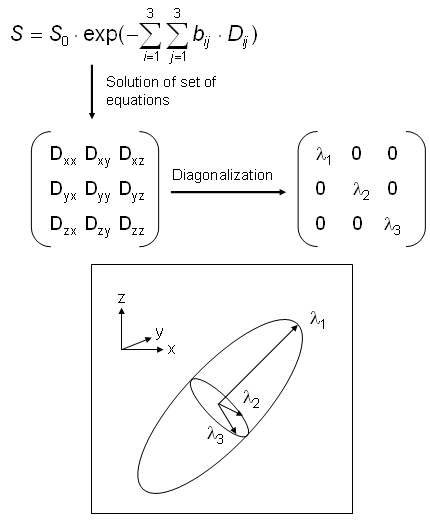 Figure 28 |
| Top |
References |
Websites:
|
| Top |
|
||||